Hot dip galvanizing is one of the most effective and durable ways to protect steel from rust and other forms of corrosion.
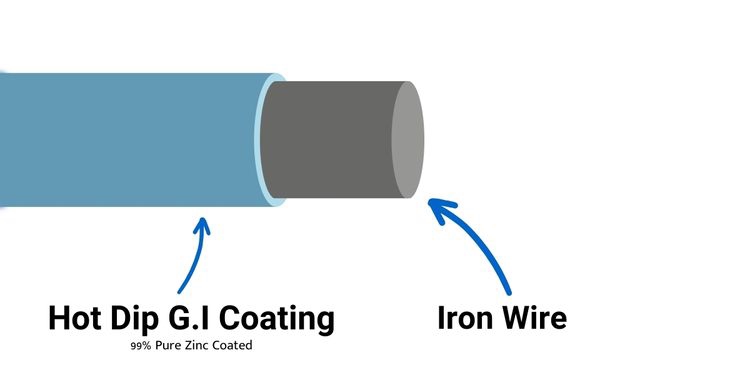
Hot-dip galvanizing is a process of coating steel with a layer of zinc to protect it from corrosion. The steel parts to be protected are submerged into a molten zinc bath at a temperature of approximately 850°F (450°C). The zinc reacts with the iron in the steel to form a metallurgically bonded zinc coating. This coating is resistant to corrosion and can last for many years, even in harsh environments.
When freshly galvanized steel is exposed to the air, it reacts with oxygen to form a very thin layer of zinc oxide powder on the surface. This layer is very thin and almost invisible.
Over the next few days, the zinc oxide reacts with hydrogen in the air to form zinc hydroxide. Zinc hydroxide is a white, powdery substance.
Over the next few months, the zinc hydroxide reacts with moisture in the air to form zinc carbonate. Zinc carbonate is a hard, durable layer that protects the underlying zinc from corrosion.
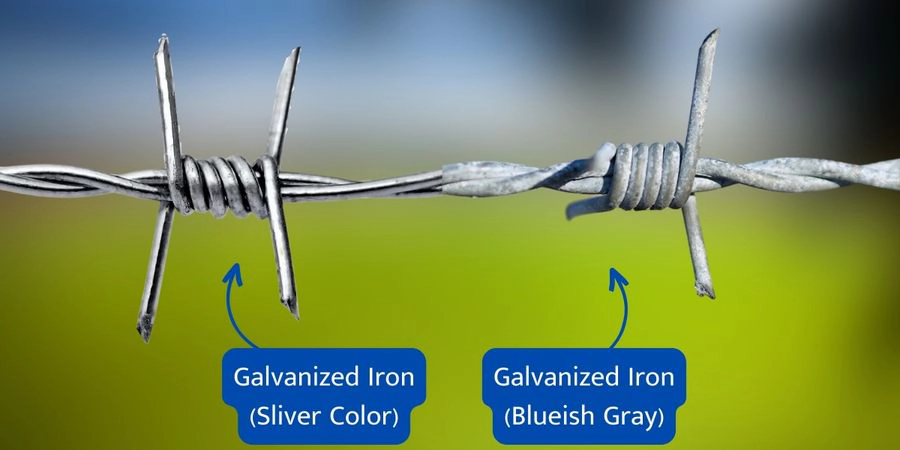
The zinc carbonate layer is called a patina. It is what gives galvanized steel its characteristic blueish gray color.
The patina layer is also what makes galvanized steel so durable. It is tightly bound to the zinc coating and protects it from corrosion, even in harsh environments.








